
Hence, the values of atomic radii given here in picometers can be converted to atomic units by dividing by 53, to the level of accuracy of the data given in this table.Ītomic radii up to zinc (30) atomic number It is often denoted by a 0 and is approximately 53 pm.

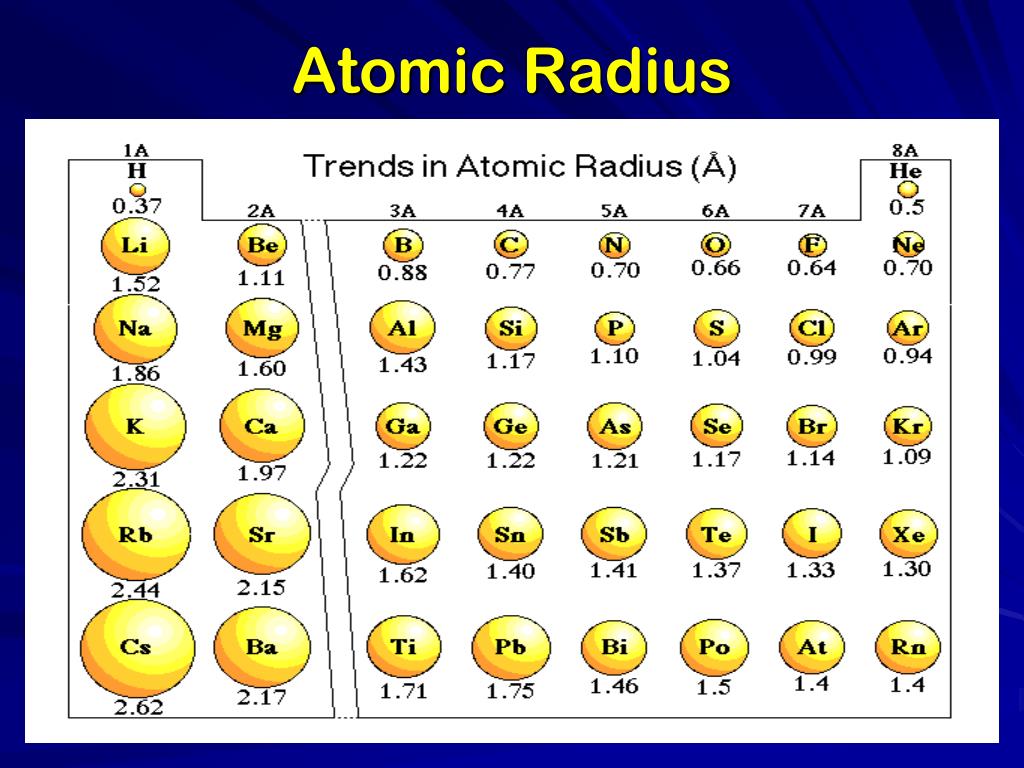
The Bohr radius is consequently known as the "atomic unit of length". Just as atomic units are given in terms of the atomic mass unit (approximately the proton mass), the physically appropriate unit of length here is the Bohr radius, which is the radius of a hydrogen atom. For more recent data on covalent radii see Covalent radius. Note: All measurements given are in picometers (pm). These trends of the atomic radii (and of various other chemical and physical properties of the elements) can be explained by the electron shell theory of the atom they provided important evidence for the development and confirmation of quantum theory. The radius increases sharply between the noble gas at the end of each period and the alkali metal at the beginning of the next period.

For instance, the radii generally decrease rightward along each period (row) of the table, from the alkali metals to the noble gases and increase down each group (column). Ītomic radii vary in a predictable and explicable manner across the periodic table. Under some definitions, the value of the radius may depend on the atom's state and context. Depending on the definition, the term may apply only to isolated atoms, or also to atoms in condensed matter, covalently bound in molecules, or in ionized and excited states and its value may be obtained through experimental measurements, or computed from theoretical models. Since the boundary is not a well-defined physical entity, there are various non-equivalent definitions of atomic radius. The atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron.


 0 kommentar(er)
0 kommentar(er)
